Dear Valued Customer,
You will no doubt be aware of the recent National Patient Safety Alert (NatPSA/2021/001/MHRA).
The alert was raised because a supplier of infusion therapy products notified the MHRA that the sterility of some of its devices could not be guaranteed, due to quality issues with its third-party sterilisation provider, Steril Milano.
B. Braun Medical Ltd would like to take this opportunity to reassure you that our products are NOT sterilised by Steril Milano and meet all requirements.
Certification of B. Braun Medical’s compliance with the appropriate governing standards is detailed below.
B. Braun Medical would also like to notify any customers that are affected by the issue outlined in this National Patient Safety Alert that our supply of Dedicated IV Pump Sets is robust, and can support redistribution, or higher set consumption within hospitals.
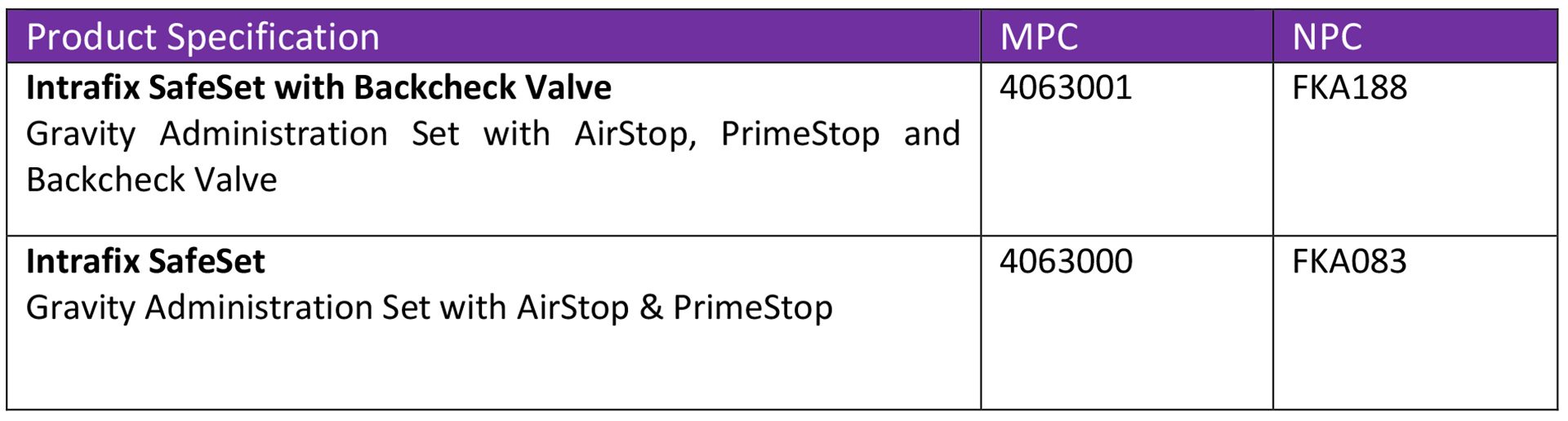
We can also offer immediate additional supply of IV Gravity Administration Sets as follows: