Advanced wound care with silver dressings
Silver dressings are the up-to-date wound care products intended to manage bioburden in acute and chronic wounds. The silver is used in various forms in the wound dressings.
Recommended use of silver dressings
Recommended use of silver dressings
Silver dressings are recommended for acute or chronic wounds with, or at risk of, a high level of bioburden or local infection. The wound infection continuum that describes the stages of impact microbes have on a wound proposes silver dressings for wounds that present localized (overt or covert), spreading or systemic infection.2)
It follows that the greater role silver dressings play is to
- reduce bioburden in infected or colonized wounds and to
- act as an antimicrobial barrier for wounds at high risk of infection or re-infection4) and not to directly promote the healing of the wound.
Note: The use of silver dressings should also be combined with other aspects of standard wound care protocols such as a holistic assessment of the patient as well as the wound, the management of underlying comorbidities and appropriate wound bed preparation.5)
The two-week challenge
Antimicrobial dressings were recommended initially for a period of two weeks, to be followed by an extensive assessment of the wound and the patient to adapt further wound management.5)
These two weeks may be used to ‘challenge’ the efficacy of silver dressings.4)
If after two weeks
- the wound has improved, but there are still signs of infection, it may be clinically legitimate to continue the application of silver dressings with further regular assessment
- the wound has improved and there aren’t any signs or symptoms of wound infection, the treatment with silver dressing should be stopped
- no improvement in the wound has been observed, the silver-dressing treatment should be stopped and an alternative treatment with a different antimicrobial agent considered.4)
Mode of action of Askina® Calgitrol®
Choosing a dressing on the basis of patient and wound needs
Askina® Calgitrol®, and in general silver dressings, are available in different forms. The choice of dressing depends on the availability of the dressings in the clinical setting and of course on the needs of the wound and the patient, e.g. exudate level, wound depth, need for conformability, odor control, ease of removal , odor control, ease of removal and safety.4)
Askina® Calgitrol®: one technology, three products
Askina® Calgitrol®: one technology, three products
The Askina® Calgitrol® range is available in three different forms:
- Askina® Calgitrol® Ag, an absorbent foam layer and a layer of silver alginate matrix
- Askina® Calgitrol® THIN, a thin layer of silver alginate matrix
- Askina® Calgitrol® Paste, an amorphous silver alginate matrix in paste form
The three dressings are easy to apply and intended to maintain a moist wound environment conducive to natural healing conditions.
Each type of Askina® Calgitrol® is recommended for different wound characteristics.
Videos
Videos to discover Askina® Calgitrol®
-
Discover the antimicrobial effects of silver ions and how Askina® Calgitrol® works.
-
How to use Askina® Calgitrol®
Askina® Calgitrol® Paste - Highly conformable, not only for small tunnels and sinuses
Askina® Calgitrol® Paste – highly conformable, not only for small tunnels and sinuses
Close contact between silver alginate matrix and the wound bed
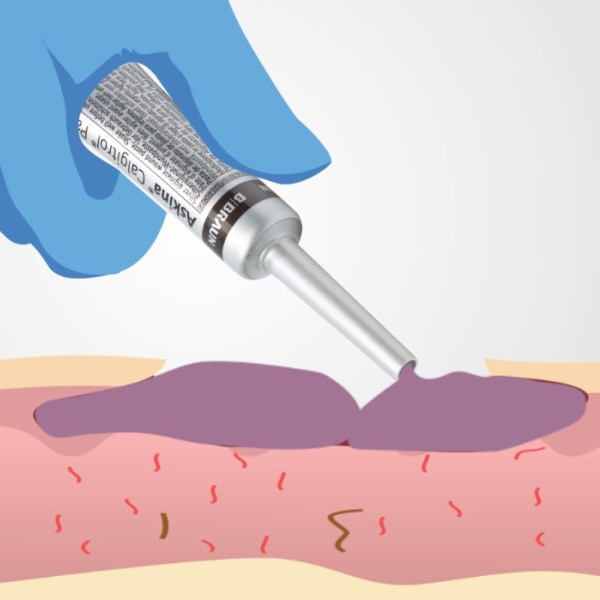
Askina® Calgitrol® Paste is an amorphous silver alginate matrix in paste form. It is highly conformable and provides extremely close contact to the wound bed.
Askina® Calgitrol® Paste for small tunnels and sinuses
This high conformability may prevent the formation of pools of exudate where bacteria might flourish. The small 15g tube comes with a long cannula to facilitate the application of the paste into small tunnels, sinuses and awkward wound shapes.8)
Optimal conformability adapts to all kinds of wound shapes
Askina® Calgitrol® Paste® may also be used for other wounds such as deep cavity or difficult-to-dress wounds.
Regarding flat, superficial, low exuding wounds Askina® Calgitrol® Paste® may be spread on the entire surface of the wound bed by using a sterile glove or a sterile spatula. It must be covered with an appropriate secondary dressing depending on the amount of exudate.
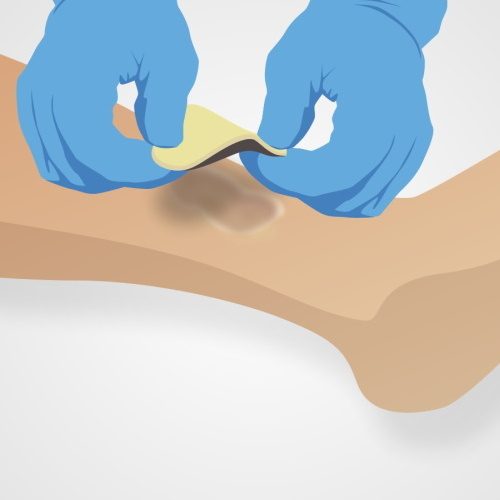
Askina® Calgitrol® Paste® should be reapplied after each dressing change (daily or up to three days depending on wound condition) or if wound exudate leakage occurs through the secondary dressing.11) Askina® Calgitrol® Paste® is easy to remove by simple rinsing with sterile saline (0.9%) or Prontosan® Wound Irrigation Solution.11)
Indications
Askina® Calgitrol® Paste is indicated for the management of partial to full thickness wounds, stage I - IV pressure ulcers, venous, arterial and neuropathic ulcers, second degree burns and donor sites.11)
Askina® Calgitrol® AG – for flat superficial highly exuding wounds
Askina® Calgitrol® Ag – for flat superficial highly exuding wounds
Exudate managment
Askina® Calgitrol® Ag is a dressing that consists of an absorbent foam layer and the silver alginate matrix. The primary function of the polyurethane foam layer is to provide absorption for the wound exudate, so no need to cover with a secondary absorbing dressing.
In contact with wound exudate, the ionic silver alginate matrix forms a soft gel.
How to use Askina® Calgitrol® Ag
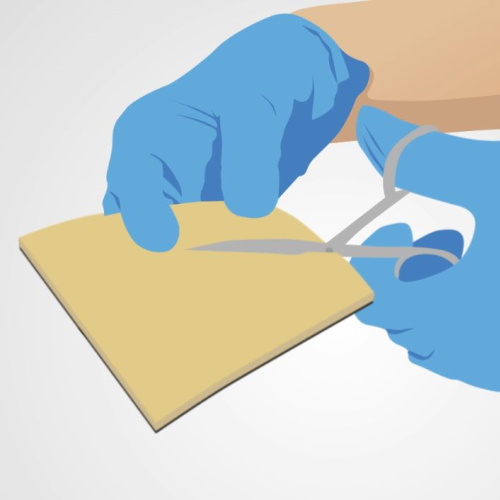
- Select the appropriate dressing size to completely cover the wound surface, ensuring 2 to 3 cm excess beyond the edges of the wound. Askina® Calgitrol® Ag can be cut to size, if needed.
- Place the dressing with the silver matrix (dark surface) facing the wound. If necessary, several dressings can be overlapped to cover very large wound areas.
- To secure Askina® Calgitrol® Ag cover it with an appropriate secondary dressing or secure with surgical tape or bandage.11)
Fewer dressing changes
Askina® Calgitrol® Ag may be left in place for up to seven days or until the dressing is saturated with wound exudate.
Indications
Askina® Calgitrol® Ag is indicated for the management of exuding partial to full thickness wounds, stage I to IV pressure sores, venous ulcers, second degree burns and donor sites.
Askina® Calgitrol® THIN – also suitable for cavity wounds
Askina® Calgitrol® THIN – also suitable for cavity wounds
Great adaptability
Askina® Calgitrol® THIN is a slim layer of silver alginate matrix.
In contact with wound exudate, the ionic silver alginate matrix forms a soft gel.
Askina® Calgitrol® THIN is recommended not only for shallow wounds but also suitable for deep cavity wounds.11) In addition, the thinness of the dressing allows to conform the dressing to irregularly shaped wounds or to wounds in awkward anatomical sites.8)
Shallow wounds
- Select the appropriate Askina® Calgitrol® THIN size that will completely cover the wound surface, ensuring 2 to 3 cm excess beyond the edges of the wound.
- Askina® Calgitrol® THIN can be cut to size, if needed, before removing the protective films.
- If necessary, several dressings can be overlapped to cover very large wound areas.
- As Askina® Calgitrol® THIN does not absorb wound exudate; it should be covered with an appropriate secondary dressing depending of the amount of exudate.
Deep cavity wounds
Pack Askina® Calgitrol® THIN into deep wounds and cover the wound with appropriate secondary dressing depending of the amount of exudate.
Fewer dressing changes
Askina® Calgitrol® THIN may be left in place for several days until the secondary dressing requires changing.
Indications
Askina® Calgitrol® THIN is indicated for the management of partial to full thickness wounds, stage 1 to IV pressure ulcers, venous ulcers, second degree burns and donor sites.
Wound care blog
Wound Care Blog
-
Prontosan® Debridement Pad | Before and After
Read MoreThe Prontosan® Debridement Pad is intended to support the soft mechanical debridement of chronic wounds in combination with Prontosan® Wound Irrigation Solution.
-
Award Winners! Integrating a Wound Cleansing Pathway into standard care.
Read MoreThe Doncaster Skin Integrity Team recently received a Bronze JWC award in the Infection and Biofilm category for their work on their local Wound Cleansing Pathway.
-
The role of Prontosan® in a pathway for biofilm based wound care
Read MoreAnita Kilroy-Findley, Clinical Lead Tissue Viability, Leicestershire Partnership NHS Trust, shares her experience of using the Prontosan in a pathway for biofilm based wound care.
References:
1) Cutting K, White R, Edmonds M. The safety and efficacy of dressing with silver - addressing clinical concerns. Int Wound J 2007; 4(2): 177-84.
2) International Wound Infection Institute (IWII) Wound infection in clinical practice. Wounds International 2016
3) Figure adapted from Molecular Expressions Web site. Michael Davidson and the Florida State University Research Foundation. https://micro.magnet.fsu.edu/ Accessed January 2012. / Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52(4):662-668.
4) International consensus. Appropriate use of silver dressings in wounds. An expert working group consensus. London: Wounds International, 2012. Available to download from: www.woundsinternational.com
5) Sibbald RG, Goodman L, Krasner DL, et al. Special considerations in Wound Bed Preparation 2011: An Update. Adv Skin Wound Care 2011; 415-36.
6) Best Practice Statement: The use of topical antiseptic/antimicrobial agents in wound management. 2nd edition. Wounds UK, London: 2011.
7) Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Review on AMR, Wellcome Trust, HM Government, 2014. Available at: http://bit.ly/1VOck4o (accessed 31.03.2017)
8) Opasanon S, Magnette A, Meuleneire F, Harding K. Askina® Calgitrol® Made Easy. Wounds International 2012; 3(1). Available from http://www.woundsinternational.com2012
9) Wounds International. Using Askina® Calgitrol® Paste for the treatment of diabetic foot infection: case studies. London: Wounds International 2013. Available from www.woundsinternational.com
10) Bowler P et al (2010) Dressing conformability and silver containing wound dressings. Wounds UK 6(2): 14–20
11) Instruction for use: Askina@ Calgitrol@ Ag, Askina@ Calgitrol@ THIN, Askina@ Calgitrol@ Paste
12) Trial C, Darbas H, Lavigne J-P, Sotto A, Simoneau G, Tillet Y, et al. Assessment of the antimicrobial effectiveness of a new silver alginate wound dressing: a RCT. J Wound Care. 2010 Jan;19(1):20–6.