No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
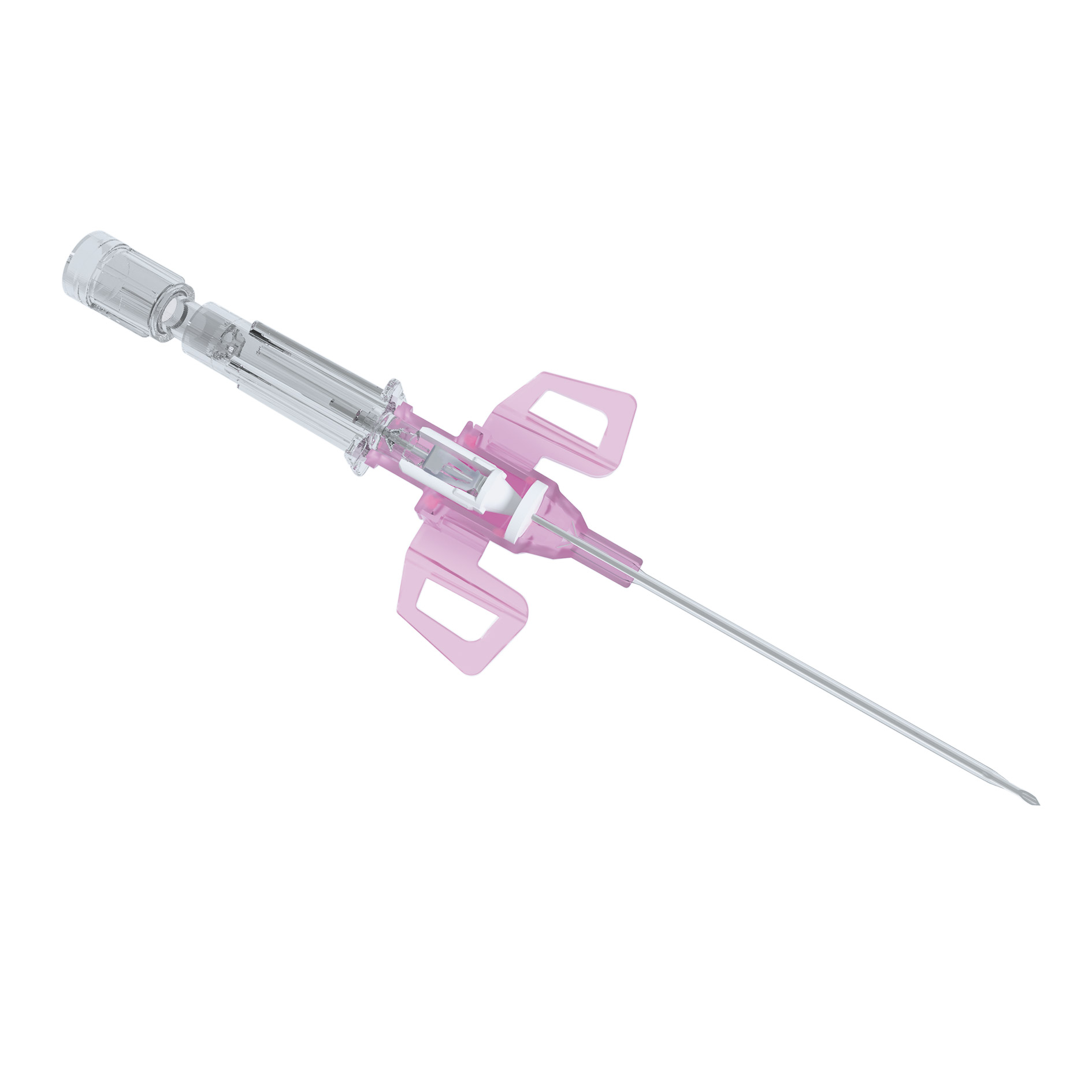
Peripheral IV Catheter
Introcan Safety® 3 is a Non-Ported Closed IV Catheter with self-activating needlestick injury protection and multi-access blood control septum that helps to protect clinicians and patients from blood exposure. Introcan Safety® 3 reduces the risk of patients acquiring a peripheral intravenous catheter related blood stream infection (PIVC-RBSI)1.
/
/
/
/
Free your hands. The multi-access blood control septum helps to protect against blood splashing and exposure during catheter insertion and while disconnecting a device from the catheter hub. The blood control septum creates a closed system helping to reduce the risk of microbial ingress.


Always be in control. The sophisticated catheter securement helps to reduce catheter movement and related complications.
Free your mind. Safety shield technology deploys automatically and cannot be bypassed, helping to prevent the risk of a needlestick injury and related infections

Thanks to our broad portfolio (G14-G24), it is the right choice for meeting all kinds of application needs, in hospital and home care.

Rely on it. Introcan Safety® 3 is indicated for use with pressure injectors up to 325psi (G24-14G)

Introcan Safety® 3 Closed IV Catheter is supplied separate to the needlefree extension. This means in the event of an unsuccessful insertion, only the PIVC and the sharp need to be disposed of, saving money and avoiding throwing away unused components (compared to catheters with an integrated needlefree extension).

Efficacy and Ease of Use of an Intravenous Catheter Designed to Prevent Blood Leakage
A Prospective Observational Trial
link
Introduction of a Non-Ported peripheral intravenous cannula with multi-use blood control septum
Offers improvements in the overall efficiency of the procedure and is clinically well accepted
link
Comparing the diagnostic accuracy of CT pulmonary angiograms
S Jones & A Shirley 2024
pdf, 204.9 KB
1. Juhlin D, Hammarskjöld F, Mernelius S, Taxbro K, Berg S. Microbiological colonization of peripheral venous catheters: a prospective observational study in a Swedish county hospital. Infect Prev Pract. 2021 Jun 7;3(3):100152. doi: 10.1016/j.infpip.2021.100152. PMID: 34458717; PMCID: PMC8379694.
2. Suzuki T. Fukuyama H. Nishiyama J. Oda M. Takahashi M. Differences in Penetration Force of Intravenous Catheters: Effect of Grinding Methods on Inner Needles of Intravenous Catheters. Tokai J Exp Clin Med. 2004; 29(4): 175-181.